- Accueil
- g heat
- Heat of reaction for, CO(g) + 1/2 O2(g)→ CO2(g)at constant V is 67.71 K cal at 17^° C. The heat of reaction at constant P at 17^° C is
Heat of reaction for, CO(g) + 1/2 O2(g)→ CO2(g)at constant V is 67.71 K cal at 17^° C. The heat of reaction at constant P at 17^° C is
4.5 (137) · € 26.50 · En Stock
Heat of reaction for, CO(g) + 1/2 O2(g)→ CO2(g)at constant V is 67.71 K cal at 17^° C. The heat of reaction at constant P at 17^° C is
Heat of reaction for- CO-g- - 1-2 O2-g- CO2-g-at constant V is-67-71 K cal at 17- C- The heat of reaction at constant P at 17- C is

heat of reaction for CU + half O2 gives CO2 at constant volume is -67.7 kilo calorie at 17 degree Celsius
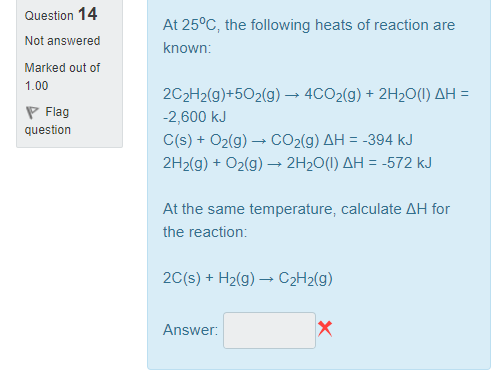
Solved Question 14 Not answered Marked out of 1.00 At 25°C

PDF) Chapter 8 Thermochemistry

Heat of reaction for; CO(g) + 1/2O2(g)→CO2(g) at constant V is - 67.71 cal 17^oC . The heat of reaction at constant P at 17^oC

Heat of reaction for, `CO(g)+1//2O_(2)(g)rarr CO_(2)(g)` at constant V is -67.71 K cal at `17^(@)C`
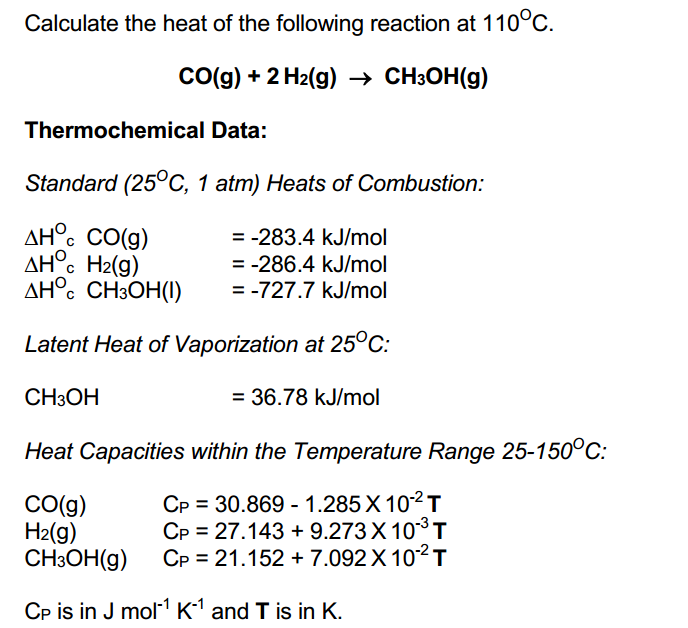
Calculate the heat of the following reaction at

The heat of formation of CO(g) and CO2(g) are ΔH=−110 and ΔH=−393kJmmol−1 respectively. What is the heat of reaction(ΔH) (in kJ mol−1) the following reaction?CO(g)+12O2(g)→CO2(g)

A 2.200-g sample of quino ne $$ (C_6H_4O_2) $$ is burned

Ch6.1 The Nature of Energy Energy – the capacity to do work or to produce heat. Law of Conservation of Energy – energy can be converted from one form to. - ppt download
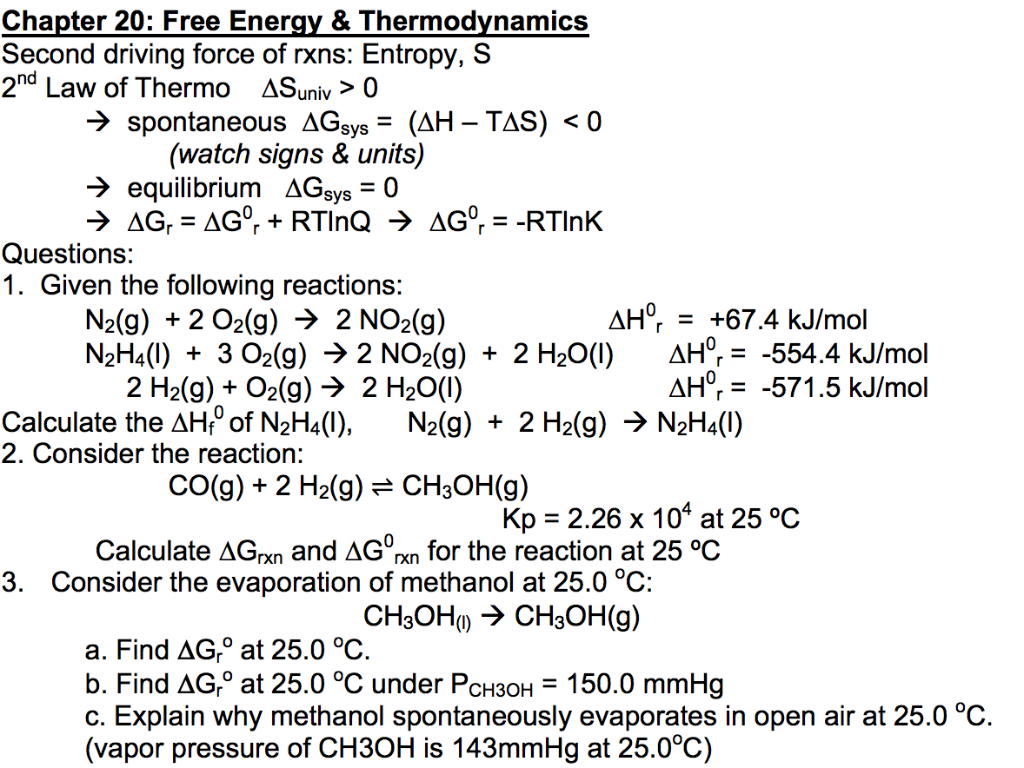
Solved 1. Given the following reactions: N2(g) + 2 O2(g) à 2

Solved When CO(g) reacts with O2(g) according to the
Heat of reaction for, CO(g)+1/2O2( g)→CO2( g) at constant V is −67.71 K..



/product/64/331/1.jpg?1426)







