- Accueil
- mcv4
- Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) vs. a licensed quadrivalent meningococcal tetanus toxoid-conjugate vaccine in meningococcal vaccine-naïve and meningococcal C conjugate vaccine
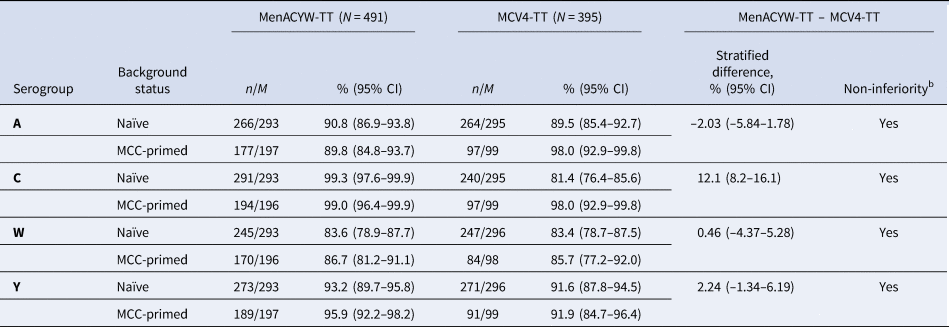
Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) vs. a licensed quadrivalent meningococcal tetanus toxoid-conjugate vaccine in meningococcal vaccine-naïve and meningococcal C conjugate vaccine
4.5 (739) · € 18.99 · En Stock

Safety and immunogenicity of a pentavalent meningococcal conjugate vaccine containing serogroups A, C, Y, W, and X in healthy adults: a phase 1, single-centre, double-blind, randomised, controlled study - The Lancet Infectious

Full article: Immunogenicity and safety of a quadrivalent meningococcal conjugate vaccine (MenACYW-TT) administered as a booster to adults aged ≥59 years: A phase III randomized study

Quadrivalent meningococcal tetanus toxoid-conjugate booster vaccination in adolescents and adults: phase III randomized study

Meningococcal ACWYX Conjugate Vaccine in 2-to-29-Year-Olds in Mali and Gambia

Full article: Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in healthy toddlers: a Phase II randomized study

A Phase II, randomized, immunogenicity and safety study of a quadrivalent meningococcal conjugate vaccine, MenACYW-TT, in healthy adolescents in the United States - ScienceDirect

Full article: Immunogenicity and safety of MenACWY-TT, a quadrivalent meningococcal tetanus toxoid conjugate vaccine recently licensed in the United States for individuals ≥2 years of age
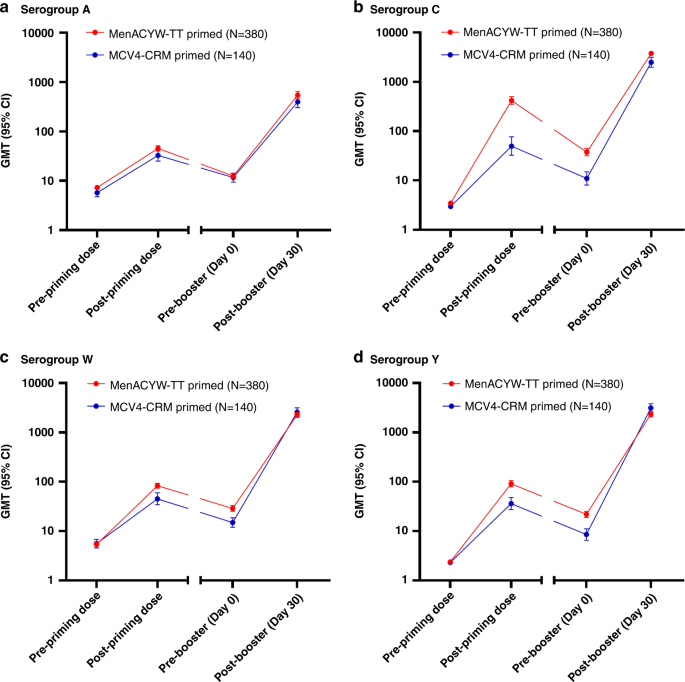
Quadrivalent meningococcal tetanus toxoid-conjugate booster vaccination in adolescents and adults: phase III randomized study

NMR Assays for Estimating the O-Acetyl Content of Meningococcal Polysaccharide Serogroup A in Quadrivalent Conjugate Vaccine Formulation

Core Concepts - Immunizations in Adults - Basic HIV Primary Care - National HIV Curriculum






.JPEG)

:watermark(cdn.texastribune.org/media/watermarks/2012.png,-0,30,0)/static.texastribune.org/media/videos/080912_Insider_Art.jpg)
